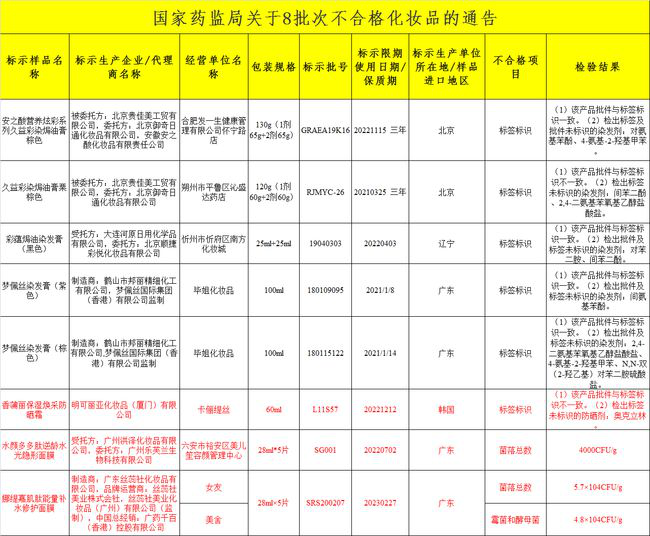
Southern Metropolis News reporter Yang Liyun Recently, the State Food and Drug Administration announced information on multiple batches of unqualified cosmetics, involving the detection of prohibited substances, the total number of colonies exceeding the standard, and the unqualified labels. A batch of South Korean imported Typhae sunscreen was on the list. Not only did it detect that the approval document was inconsistent with the label, but also the sunscreen that was not marked on the label was detected.
 The above-mentioned substandard products-related enterprises violated the provisions of the "Regulations on Hygiene Supervision of Cosmetics" and other relevant laws and regulations. The State Drug Administration requires the drug regulatory authorities of Anhui, Tibet, and Shanxi provinces (districts) to order relevant business units to immediately take measures such as removing the shelves to control risks, and to investigate and deal with violations discovered in accordance with the law.
The above-mentioned substandard products-related enterprises violated the provisions of the "Regulations on Hygiene Supervision of Cosmetics" and other relevant laws and regulations. The State Drug Administration requires the drug regulatory authorities of Anhui, Tibet, and Shanxi provinces (districts) to order relevant business units to immediately take measures such as removing the shelves to control risks, and to investigate and deal with violations discovered in accordance with the law.
5 batches of hair dye products are unqualified
One of the batches was sold in a pharmacy
The "Notice on 2 Batches of Detected Prohibited Substance Cosmetics" and "Notice on 8 Batches of Unqualified Cosmetics" recently issued by the State Food and Drug Administration show that multiple batches of hair dyes, sunscreens, and facial masks are on the list.
Among them, up to 5 batches are hair dye products, all of which are related to unqualified labels, including: the nominal Beijing Yuqi Ritong Cosmetics Co., Ltd. commissioned by Beijing Guijiamei Industry and Trade Co., Ltd. The company’s Jiuyi dyeing and baking cream chestnut brown, and Beijing Yuqi Ritong Cosmetics Co., Ltd. and Anzhi Acid Cosmetics Co., Ltd. commissioned by Beijing Guijiamei Industry and Trade Co., Ltd. to produce anzhi acid nutrition and colorful series of Jiuyi color dyeing and baking oil Cream brown and so on.
A batch of South Korea imported Xiangpree sunscreen is on the list
It is worth mentioning that a batch of South Korean imports of Sankriya Cosmetics (Xiamen) Co., Ltd. produced a lot of moisturizing and rejuvenating sunscreen.
In addition to the inspection of the approval document and the label identification inconsistency, the product also detected a sunscreen agent that was not identified on the label: octopine. It is understood that octocrelin is a water-soluble broad-spectrum ultraviolet absorber, usually used with other sunscreen agents, the maximum amount of addition is 10% as defined by domestic laws and regulations.



